Dielectric Protocol Advancements Push Energy Density to New Heights in Li-Metal Pouch Cells
Key Factors in Battery Energy Conversion Efficiency
The Electrode-Electrolyte interface is a key factor in battery energy conversion efficiency. Over recent years, considerable attention has been devoted to modifying this interface to improve the performance and energy density of Lithium-Metal Batteries (LMBs).
Promise of Lithium-Metal Batteries
Lithium-Metal Batteries (LMBs) offer a promising alternative by incorporating Li-metal anodes, in contrast to the graphite-based anodes commonly used in Lithium-ion batteries (LiBs). This technology presents the potential for substantially higher energy densities and faster charging times.
Current Limitations of LMBs
While LMBs hold great promise, current iterations face considerable limitations, including high production costs, poor Coulombic efficiency, and the development of Li dendrites during charging. These dendritic formations on anodes not only reduce battery performance but also increase the likelihood of overheating and fire.
Addressing Key Limitations of LMBs
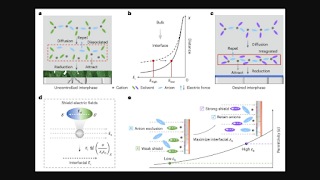
One potential approach to address the key limitations of LMBs involves regulating the Li⁺ solvation structure and developing new electrolytes to promote the formation of a Solid-Electrolyte Interphase (SEI) and stabilize the electrode/electrolyte interface. While numerous studies focus on these strategies, few have investigated the role of the dielectric environment in stabilizing or destabilizing this interface.
Recent Research and Dielectric Protocol
Researchers from Zhejiang University, along with several other Chinese institutions, have recently explored this topic. Their work, detailed in Nature Energy, propose a dielectric protocol aimed at mitigating issues with LMBs, potentially improving both their safety and operational reliability.
Expert Insights
Xiulin Fan's Perspective
Xiulin Fan, a co-author of the paper, shared with TechXplore, "As the markets for electric vehicles and energy storage expand, so will the demand for Lithium-ion Batteries (LIBs). However, achieving a Low-Carbon or Carbon-Free economy requires batteries that outperform current LIBs. This means we need energy storage technologies with densities greater than 500 Wh/kg, which would extend the operational time of electric devices on a single charge. while Lithium Metal Batteries (LMBs) with metal electrodes offer promise, they face challenges such as premature degradation in both laboratory and industrial settings. Our main objective was to develop- LMBs that offer both longevity and high energy density."
Design Methodology and Protocol
Impact of Interfacial Electric Field
According to the researcher's paper, their design methodology for LMBs incorporates the impact of the interfacial electric field, which can be modulated by the battery's dielectric materials, on the electrode/electrolyte interface. By managing the dielectric medium, their protocol maintains the integrity of cation-anion interactions and facilitates SEI formation by exposing the anion-rich electrolyte to the electric field at the interface.
Dielectric Protocol Details
Fan described how the dielectric protocol calls for placing cation-anion pairs in a non-solvating solvent characterized by a high dielectric constant. This strategy protects the pairs from being disrupted by the electric field, forming an anion-dense region near the electrode-electrolyte interface. This design facilitates the decomposition of anions at the interface, thus enhancing the durability of the interfacial chemistry for lithium deposits in Li-metal pouch cells.
Cation-Anion Pair Distribution
According to Zhang, Li, and their team, cation-anion pairs at charged interfaces exhibit a periodic oscillatory distribution. Lower oscillation amplitudes contribute to increased electrolyte degradation and elevated surface impedance. Their proposed dielectric protocol addresses these issues by maintaining high oscillation amplitudes to preserve cation-anion coordination at the interfaces.
Results and Future Directions
Achievements with New Protocol
By applying their newly proposed protocol, the team created an ultra-lean electrolyte (1g Ah¯¹) and evaluated it in lithium-metal pouch cells. The tested cells showed an outstanding energy density of 500 Wh kg¯¹.
Insights and Future Research
Fan explained that this research unveils the spatial distribution of anions and cations at the charged electrode-electrolyte interface. This insight enables the adjustment of interfacial properties through precise electrolyte composition, potentially enhancing battery performance.
Broader Implications
This research team's dielectric-mediated strategy may soon serve as a model for other groups aiming to develop advanced electrolytes for LMBs. Such collaborative efforts could enhance the creation of more dependable high-density battery technologies.
Safety and Future Goals
"Fan added that while Li-metal batteries offer high energy density, they also bring about severe safety concerns like fires and explosions. Our future research will aim to improve the cycle stability of these batteries in realistic conditions, striving to create a technology that balances high energy density with safety."
Source
Labels: Batteries, Dielectric Environment, Energy